Research interest
INORGANIC CHEMISTRY
The ultimate aim of this research is to synthesize and characterize
new metal complexes based on ruthenium, copper, zinc, vanadium and
gallium the goal to establish their ability to disrupt cell
proliferation and of optimizing their ability to interact with
biological macro-molecules. The key roles that transition metal ions
play in biological systems are well established and transition metal
complexes are finding increasing usage in clinical medicine
including oncology. The platinum-based anticancer drugs cisplatin
and carboplatin are the most well-known. However, it is important to
synthesize new classes of anticancer agents and the design of new
potential drugs is increasingly focused on ruthenium complexes. A
new class of organometallic ruthenium complexes, [(arene)Ru(XY)Cl]+
(XY = ethylenediamine) have been reported to show significant
cytotoxic activity. Other types of these complexes containing a
variety of XY ligand systems have also been investigated. The
mechanism of cytotoxicity for ruthenium compounds has not been
established. Nucleic acids, particularly DNA, are considered a high
probability target. Proteins, including the topoisomerase enzymes
are also certainly potential targets.
My research is designed to investigate the chemical and biological
properties of organometallic and inorganic ruthenium (primarily)
complexes. We have already establish that we can prepare complexes
of the type [Ru(arene)(XY)Cl]+ and that these complexes
can bind to DNA in a yet-determined binding mode.
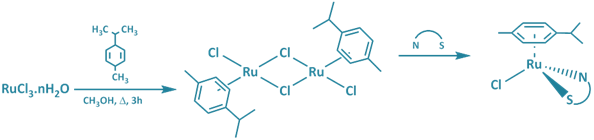
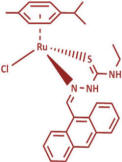
We carry out a number of experiments in order to probe the mode as
well as strength of binding to the nucleic acid. The methods to be
used include absorption spectroscopy, thermal denaturation studies,
electrochemical and viscometric measurements. Mechanistic studies to
determine kinetic and thermodynamic data is also derived. We also
investigate the potential of topoisomerase II as an anticancer
target for our complexes and use models such as ubiquitin and human
serum albumin to probe the potential to interact with proteins.
In these complexes we use two classes of ligand (XY):
thiosemicarbazones (TSCs) and curcuminoids (CCs).
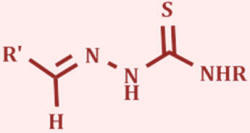
Thiosemicarbazones have a versatile range of biological effects such
as antiviral, antineoplastic and anticancer properties, and we
expect to see improved biological activity by coupling with the
organometallic ruthenium moiety.
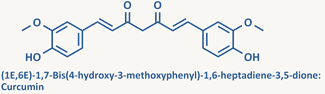
Structurally, curcuminoids are
1,7-diaryl-l.6-heptadiene-3,5-diones
in which two oxy-substituted aryl moieties are linked together
through a seven-carbon chain. They are generally analogs of
curcumin.
Curcumin is an important natural phytochemical found in the rhizomes
of
Curcuma longa
or
turmeric and it
possesses a variety of remarkable pharmacological activity including
anti-inflammatory, anti-carcinogenic, anti-oxidant activity and even
anti-amyloid activity.
Synthetic curcuminoids are believed to be more potent
anti-carcinostatic agents. These compounds therefore seem to
excellent ligand systems for metal complexes and we are particularly
interested in the potential synergy to be derived by coupling to a
ruthenium organometallic fragment.
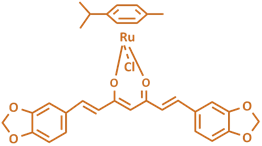
We are also interested in other classes of (inorganic) ruthenium
complexes
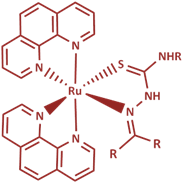
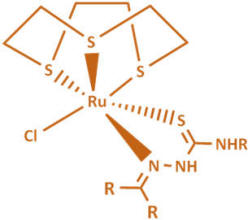
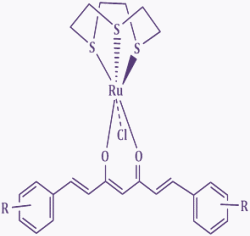
Other metal under investigation includes gallium which has long been
used in medical applications and the antitumor properties of some
gallium complexes have been known since the 1960s. Vanadium, copper
and zinc complexes of curcuminoids are also currently being studied.
ENVIRONMENTAL CHEMISTRY
My interest in environmental chemistry stems from my inorganic
chemistry background. My postdoctoral training was in the design of
organometallic compounds that can be used as catalyst in
environmentally benign solvent. I want to go back to this area by
investigating the uses of ionic liquids as the benign solvents. The
compounds have over the last decade or so been touted as green
alternatives to organic solvents. Their lack of vapor pressure is
one reason for the optimism. I aim to investigate simple ionic
liquids based on imidazole and to use my ruthenium complexes as
catalysts.
I am also interested in investigating the prevalence and behavior of
pharmaceuticals and personal care products in the environment. This
is a new area of interest that I am currently trying to develop.